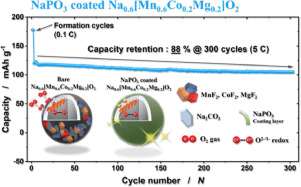
Herein, the surface of the P3-Na0.6[Mn0.6Co0.2Mg0.2]O2 cathode material is fortified by introducing an ionic-conducting sodium-phosphate nanolayer (NaPO3, ≈10-nm thickness). This layer facilitates Na+-ion diffusion owing to its sufficiently high ionic conductivity (≈10–6 S cm–1). Moreover, the NaPO3 coating layer prevents the precipitation of surface byproducts generated from reaction with the electrolyte. The NaPO3-coated P3-Na0.6[Mn0.6Co0.2Mg0.2]O2 electrode can thus retain over 80% of the first capacity after 200 cycles not only at 0.1C but also at a high rate (5C), with a capacity retention of 88% after 300 cycles. Reversible transition-metal and oxygen redox are evidenced by X-ray absorption near-edge spectroscopy, X-ray photoelectron spectroscopy, time-of-flight secondary-ion mass spectroscopy, and operando differential electrochemical mass spectroscopy, which reveal mitigated surface-byproduct formation. These findings demonstrate the possibility of the use of oxygen redox for high-energy SIBs, ensuring long term cyclability.
