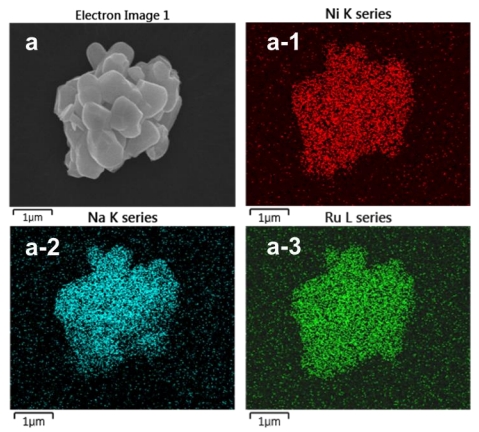
Herein, stable cationic and anionic redox in an O3-type layered Na[Ni2/3Ru1/3]O2 cathode for sodium-ion batteries (SIBs) is revealed. Density functional theory (DFT) calculation shows that the electron density features change in density of state with mixing of delocalized valence states as well as localized deeper energy states of O(p), Ni(d), and Ru(d) for the highly desodiated Na1−x[Ni2/3Ru1/3]O2 electrode, revealing the covalent characteristic of the transition metal (TM)O and TMTM bonds in the charged system. These properties lead to cycling stability for 200 cycles, with ≈79% of the capacity retained at a rate of 1C (210 mA g−1). Operando X-ray diffraction, X-ray absorption spectroscopy, and DFT calculations reveal the reversible electrochemical activity of the Ni2+/Ni3+ and O2−/O1− redox reactions, which are sustainable throughout the cycles. In addition, no loss of oxygen from the crystal structure of Na[Ni2/3Ru1/3]O2 occurs according to differential electrochemical mass spectrometry. The findings provide additional insight into the complex mechanism of the oxygen redox activity of high-capacity O3-type cathode materials for SIBs, encouraging further studies on their development.
