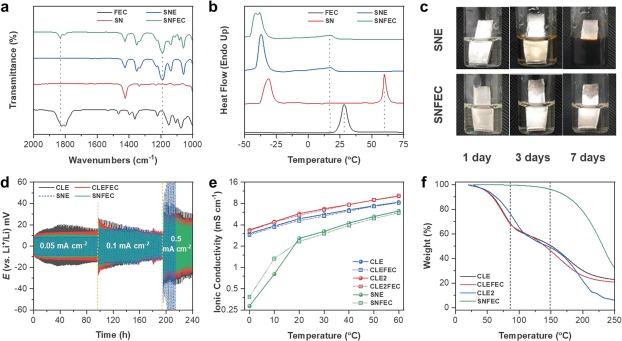
Sn-based materials have been highlighted as promising anodes for next-generation batteries; however, such alloying-based anodes suffer from gradual cell degradation caused by the associated large volume changes, leading to particle pulverization and an unstable solid electrolyte interphase (SEI). A key to constructing a stable SEI as well as effectively buffering the large volume expansion is to establish appropriate electrolyte conditions. In this work, to induce the formation of a favorable SEI layer, a Sn anode is paired with an electrolyte composed of eutectic succinonitrile (SN) combined with fluoroethylene carbonate (FEC) for the first time. Whereas conventional electrolytes are prone to form organic SEI components, which are vulnerable to large volume changes, the combination of SN and FEC enables the formation of a mechanically rigid SEI that is mainly composed of favorable inorganics such as LiF and Li3N. The synergistic effects of SN and FEC result in superior cycle performance of the Sn anode with improved efficiency compared with that achieved using conventional carbonate electrolytes. The modified SEI layer was closely investigated using various surface analysis techniques, with the individual effects of SN and FEC extracted. The rational design of the electrolyte provides a simple yet effective approach to enhance the electrochemical performance of Sn anodes and can be further extended to other alloying-based anode materials.
