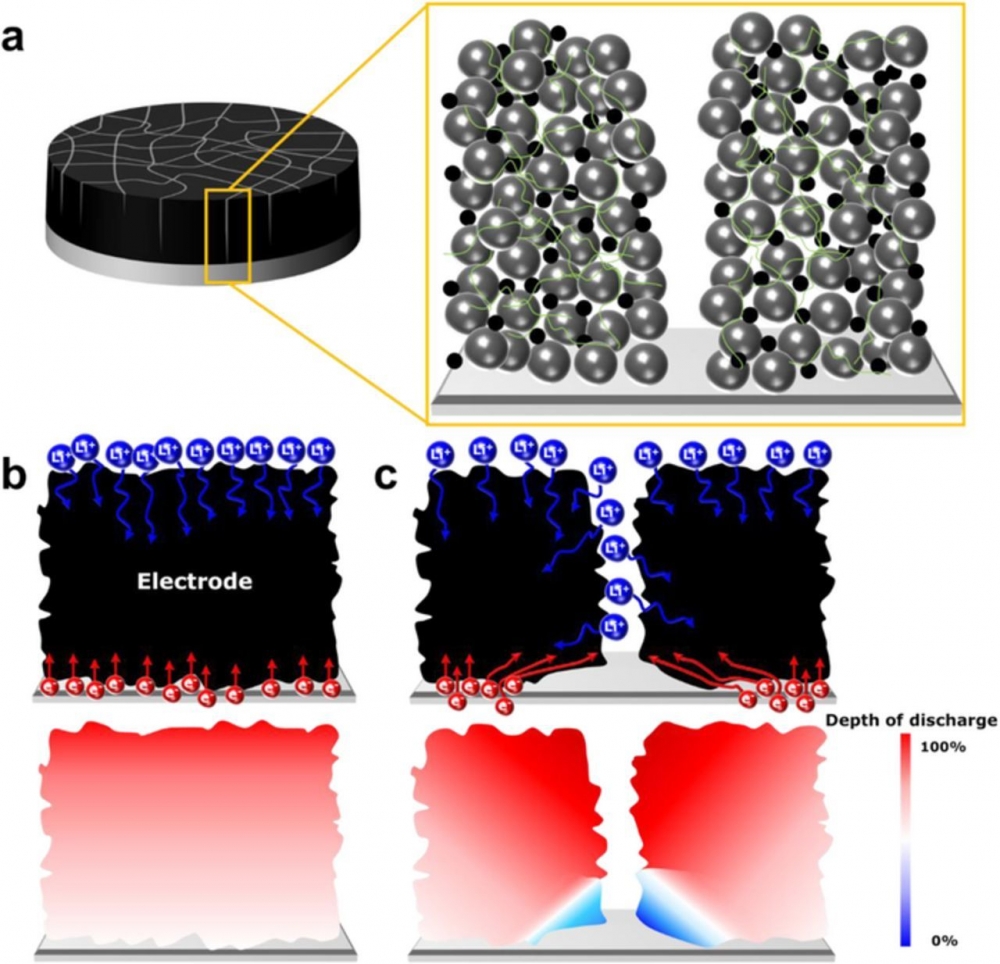
Increasing electrode thickness and loading can help Li-ion batteries achieve higher energy densities, but the resulting decay in electrochemical performance at elevated rates remains a significant challenge. In order to design an optimal thick electrode, understanding how performance loss occurs is necessary. While it is known that both ionic and electronic conductivity contribute to rate performance, we observed a stronger correlation between electronic conductivity and electrochemical performance of electrodes at a loading of >25 mg/cm2 under C/3 to 1C, rates most relevant to electric vehicle applications. To illustrate this effect, we explore the mud-cracking phenomenon during electrode fabrication to obtain narrow, vertical channels which reduce electrode tortuosity, and therefore decrease the liquid phase ionic resistance in thick electrodes. Variation in crack densities enables us to systematically investigate the effects of ionic and electronic conductivity on electrochemical performance in electrodes with identical overall porosity and composition. Rate and cycling performances of mud-cracked thick electrodes have stronger correlations with electronic conductivity than ionic conductivity. These findings shed new light on the relative importance of electronic versus ionic conductivities, arguing for the need to further optimize electronic conduction in thick electrodes when they are cycled in conditions relevant to electric vehicle applications.
