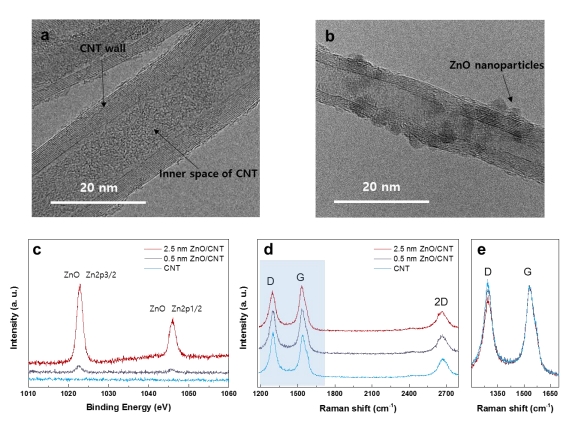
Li-O2 batteries are promising next-generation energy storage systems because of their exceptionally high energy density (≈3500 W h kg−1). However, to achieve stable operation, grand challenges remain to be resolved, such as preventing electrolyte decomposition and degradation of carbon, a commonly used air electrode in Li-O2 batteries. In this work, using in situ differential electrochemical mass spectrometry, it is demonstrated that the application of a ZnO coating on the carbon electrode can effectively suppress side reactions occurring in the Li-O2 battery. By probing the CO2 evolution during charging of 13C-labeled air electrodes, the major sources of parasitic reactions are precisely identified, which further reveals that the ZnO coating retards the degradation of both the carbon electrode and electrolyte. The successful suppression of the degradation results in a higher oxygen efficiency, leading to enhanced stability for more than 100 cycles. Nevertheless, the degradation of the carbon electrode is not completely prevented by the coating, because the Li2O2 discharge product gradually grows at the interface between the ZnO and carbon, which eventually results in detachment of the ZnO particles from the electrode and subsequent deterioration of the performance. This finding implies that surface protection of the carbon electrode is a viable option to enhance the stability of Li-O2 batteries; however, fundamental studies on the growth mechanism of the discharge product on the carbon surface are required along with more effective coating strategies.
