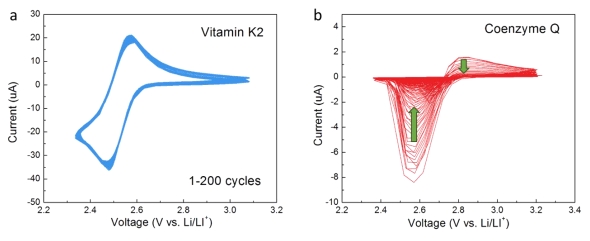
Governing the fundamental reaction in lithium–oxygen batteries is vital to realizing their potentially high energy density. Here, novel oxygen reduction reaction (ORR) catalysts capable of mediating the lithium and oxygen reaction within a solution-driven discharge, which promotes the solution-phase formation of lithium peroxide (Li2O2), are reported, thus enhancing the discharge capacity. The new catalysts are derived from mimicking the biological redox mediation in the electron transport chain in Escherichia coli, where vitamin K2 mediates the oxidation of flavin mononucleotide and the reduction of cytochrome b in the cell membrane. The redox potential of vitamin K2 is demonstrated to coincide with the suitable ORR potential range of lithium–oxygen batteries in aprotic solvent, thereby enabling its successful functioning as a redox mediator (RM) triggering the solution-based discharge. The use of vitamin K2 prevents the growth of film-like Li2O2 even in an ether-based electrolyte, which has been reported to induce surface-driven discharge and early passivation of the electrode, thus boosting the discharge capacity by ≈30 times. The similarity of the redox mediation in the biological cell and lithium–oxygen “cell” inspires the exploration of redox active bio-organic compounds for potential high-performance RMs toward achieving high specific energies for lithium–oxygen batteries.
