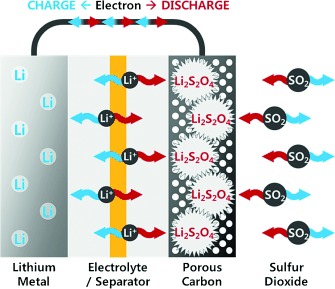
Primary Li–SO2 batteries offer a high energy density in a wide operating temperature range with exceptionally long shelf life and have thus been frequently used in military and aerospace applications. Although these batteries have never been demonstrated as a rechargeable system, herein, we show that the reversible formation of Li2S2O4, the major discharge product of Li–SO2 battery, is possible with a remarkably smaller charging polarization than that of a Li–O2 battery without the use of catalysts. The rechargeable Li–SO2 battery can deliver approximately 5400 mAh g−1 at 3.1 V, which is slightly higher than the performance of a Li–O2 battery. In addition, the Li–SO2 battery can be operated with the aid of a redox mediator, exhibiting an overall polarization of less than 0.3 V, which results in one of the highest energy efficiencies achieved for Li–gas battery systems.