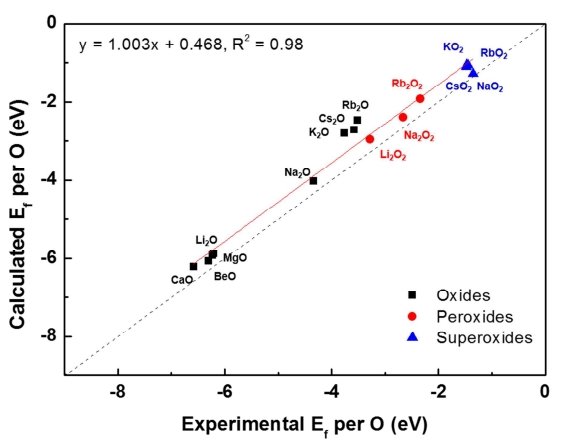
Li–oxygen and Na–oxygen batteries are some of the most promising next-generation battery systems because of their high energy densities. Despite the chemical similarity of Li and Na, the two systems exhibit distinct characteristics, especially the typically higher charging overpotential observed in Li–oxygen batteries. In previous theoretical and experimental studies, this higher charging overpotential was attributed to factors such as the sluggish oxygen evolution or poor transport property of the discharge product of the Li–oxygen cell; however, a general understanding of the interplay between the discharge products and overpotential remains elusive. Here, we investigated the charging mechanisms with respect to the oxygen evolution reaction (OER) kinetics, charge-carrier conductivity, and dissolution property of various discharge products reported in Li–oxygen and Na–oxygen cells. The OER kinetics were generally faster for superoxides (i.e., LiO2 and NaO2) than for peroxides (i.e., Li2O2 and Na2O2). The electronic and ionic conductivities were also predicted to be significantly higher in superoxide phases than in peroxide phases. Moreover, systematic calculations of the dissolution energy of the discharge products in the electrolyte, which mediate a solution-based OER reaction, revealed that the superoxide phases, particularly NaO2, exhibited markedly low dissolution energy compared with the peroxide phases. These results imply that the formation of superoxides instead of peroxides during discharge may be the key to improving the energy efficiency of metal–oxygen batteries in general.
