Rational design of redox mediators for advanced Li-O2 batteries
- Year
- 2016
- Author
- Hee-Dae Lim, Byungju Lee, Yongping Zheng, Jihyun Hong, Jinsoo Kim, Hyeokjo Gwon, Youngmin Ko, Minah Lee, Kyeongjae Cho, Kisuk Kang
- Journal
- Nature Energy
- Publisher
- Nature Publishing Group
- Vol
- 1
- Page
- 16066
- Link
- https://www.nature.com/articles/nenergy201666 708회 연결
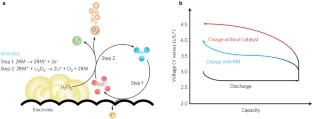
The rapid growth of portable energy demands has increased interest in next-generation batteries with high energy densities. Among several post-lithium ion battery candidates, Li–O2 batteries have attracted tremendous attention because of their exceptionally high theoretical energy density ( ∼3,500 Wh kg−1)1,2,3,4. The absence of a transition metal and the use of abundant resources of oxygen in the electrochemical reaction further make this chemistry appealing as a promising future battery system5,6,7. However, the present state of Li–O2 batteries involves the critical limitations of low cycle life and high charging overpotential (low energy efficiency) during cycling8,9,10. Although the cycle life and high overpotential are believed to be correlated to each other to some extent, the origin of the latter is often attributed to several factors in the decomposition process of Li2O2, such as the sluggish oxygen evolution from the Li2O2 crystal, low electrical conductivity, and formation/decomposition of unwanted products, including Li2CO3 (refs 11,12,13,14). Furthermore, the solid discharge product (Li2O2) in Li–O2 batteries is irregularly formed on the electrode; thus, the porous air cathode is easily clogged, hindering the efficient transport of oxygen and ions, causing further overpotential15,16. Addressing these issues and enhancing the energy efficiency by decreasing the overpotential are indispensable in taking advantage of the high energy density of the Li–O2 battery chemistry.