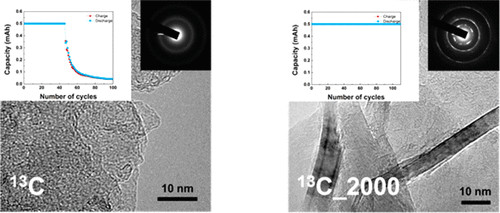
The Li–O2 battery is capable of delivering the highest energy density among currently known battery chemistries and is thus regarded as one of the most promising candidates for emerging high-energy-density applications such as electric vehicles. Although much progress has been made in the past decade in understanding the reaction chemistry of this battery system, many issues must be resolved regarding the active components, including the air electrode and electrolyte, to overcome the presently insufficient cycle life. In this work, we demonstrate that the degradation kinetics of both the air electrode and electrolyte during cycles can be significantly retarded through control of the crystallinity of the carbon electrode, the most frequently used air electrode in current Li–O2 batteries. Using 13C-based air electrodes with various degrees of graphitic crystallinity and in situ differential electrochemical mass spectroscopy analysis, it is demonstrated that, as the crystallinity increases in the carbon, the CO2 evolution from the cell is significantly reduced, which leads to a 3-fold enhancement in the cyclic stability of the cell.
