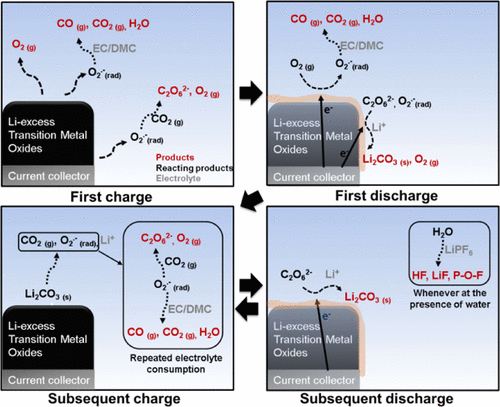
The high capacity of the layered Li–excess oxide cathode is always accompanied by extraction of a significant amount of oxygen from the structure. The effects of oxygen on the electrochemical cycling are not well understood. Here, the detailed reaction scheme following oxygen evolution was established using real-time gas analysis and ex situ chemical analysis of the surface of the electrodes. A series of electrochemical/chemical reactions involving oxygen radicals constantly produced and decomposed lithium carbonate during cell operation. Moreover, byproducts, including water, affected the cycle life and rate capability: hydrolysis of the electrolyte salt formed hydrofluoric acid that attacked the surface of the electrode. This finding implies that protection of the electrode surface from damage, for example, by a coating or removal of oxygen radicals by scavengers, will be critical to widespread usage of Li–excess transition metal oxides in rechargeable lithium batteries.