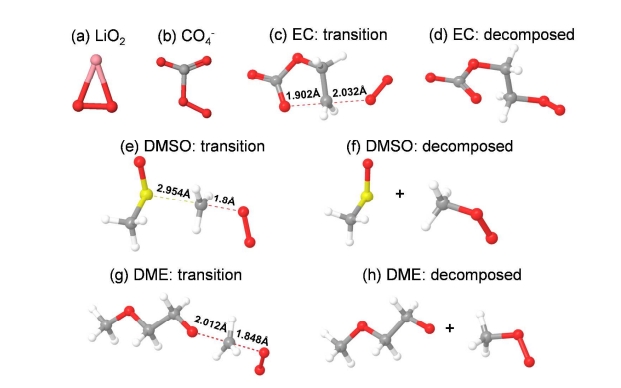
Lithium–oxygen chemistry offers the highest energy density for a rechargeable system as a “lithium–air battery”. Most studies of lithium–air batteries have focused on demonstrating battery operations in pure oxygen conditions; such a battery should technically be described as a “lithium–dioxygen battery”. Consequently, the next step for the lithium–“air” battery is to understand how the reaction chemistry is affected by the constituents of ambient air. Among the components of air, CO2 is of particular interest because of its high solubility in organic solvents and it can react actively with O2–•, which is the key intermediate species in Li–O2 battery reactions. In this work, we investigated the reaction mechanisms in the Li–O2/CO2 cell under various electrolyte conditions using quantum mechanical simulations combined with experimental verification. Our most important finding is that the subtle balance among various reaction pathways influencing the potential energy surfaces can be modified by the electrolyte solvation effect. Thus, a low dielectric electrolyte tends to primarily form Li2O2, while a high dielectric electrolyte is effective in electrochemically activating CO2, yielding only Li2CO3. Most surprisingly, we further discovered that a high dielectric medium such as DMSO can result in the reversible reaction of Li2CO3 over multiple cycles. We believe that the current mechanistic understanding of the chemistry of CO2 in a Li–air cell and the interplay of CO2 with electrolyte solvation will provide an important guideline for developing Li–air batteries. Furthermore, the possibility for a rechargeable Li–O2/CO2 battery based on Li2CO3 may have merits in enhancing cyclability by minimizing side reactions.
