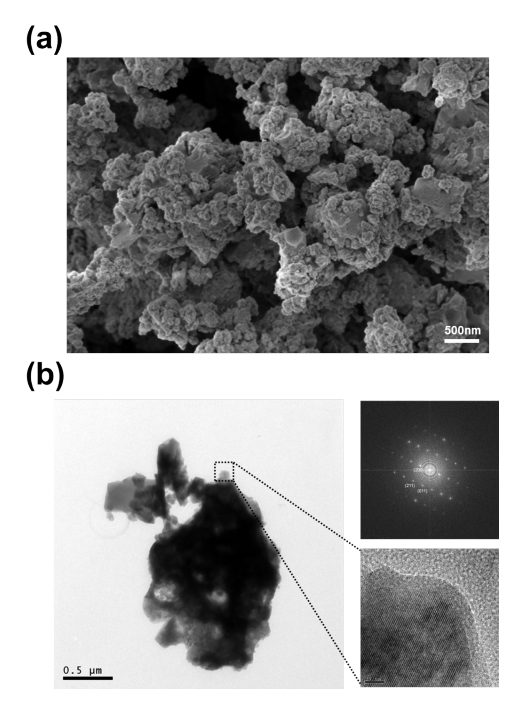
Compounds with a mixed polyanion framework have recently gained attention as a new class of compounds for material exploration. The potential tunability of the structure by using various combinations of polyanions can potentially lead to a novel cathode. However, the redox reaction in complex structures often involves complex structural evolutions during the electrochemical reaction, which require careful analysis. We investigated the electrochemical mechanism of NaxFe3(PO4)2(P2O7) (1 ≤ x ≤ 4), which was recently proposed as a promising mixed-polyanion cathode for Na rechargeable batteries, using first principles calculations and experiments. We discovered that the de/sodiation of the NaxFe3(PO4)2(P2O7) electrode occurs via a one-phase reaction with a reversible Fe2+/Fe3+ redox reaction and accompanies an exceptionally small volumetric change of less than 4%. Na ion intercalation usually induces a large volumetric change in conventional systems; therefore, this small volume change is unusual and was attributed to the open framework of polyanion compounds with P2O7 dimers that are capable of rotating and distorting to accommodate the structural change. Structural robustness was further observed at even highly charged states at temperatures as high as 530 °C from in situ X-ray diffraction (XRD) and differential scanning calorimetry (DSC). We believe that the improved understanding of the electrochemical mechanism provided here will expedite the optimization of the new Na4Fe3(PO4)2(P2O7) electrode for Na rechargeable batteries.
