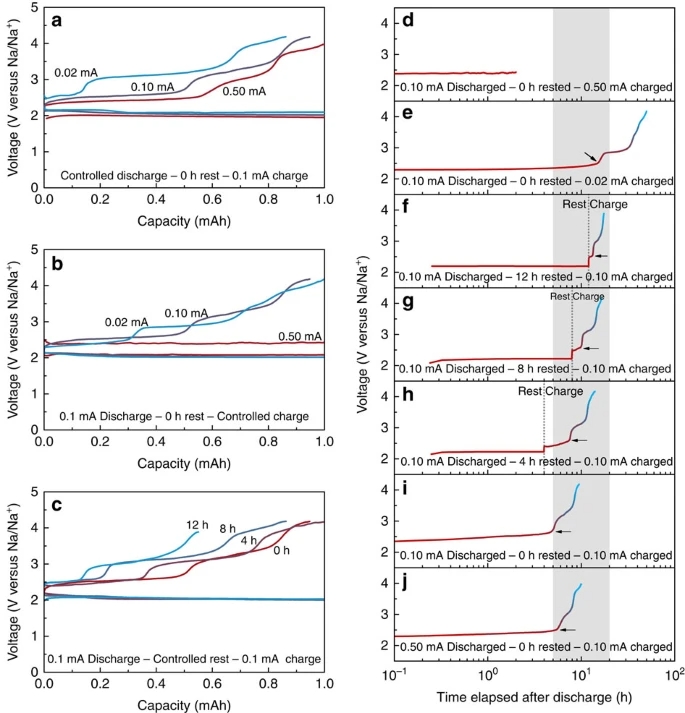
With the demand for high-energy-storage devices, the rechargeable metal–oxygen battery has attracted attention recently. Sodium–oxygen batteries have been regarded as the most promising candidates because of their lower-charge overpotential compared with that of lithium–oxygen system. However, conflicting observations with different discharge products have inhibited the understanding of precise reactions in the battery. Here we demonstrate that the competition between the electrochemical and chemical reactions in sodium–oxygen batteries leads to the dissolution and ionization of sodium superoxide, liberating superoxide anion and triggering the formation of sodium peroxide dihydrate (Na2O2·2H2O). On the formation of Na2O2·2H2O, the charge overpotential of sodium–oxygen cells significantly increases. This verification addresses the origin of conflicting discharge products and overpotentials observed in sodium–oxygen systems. Our proposed model provides guidelines to help direct the reactions in sodium–oxygen batteries to achieve high efficiency and rechargeability.
