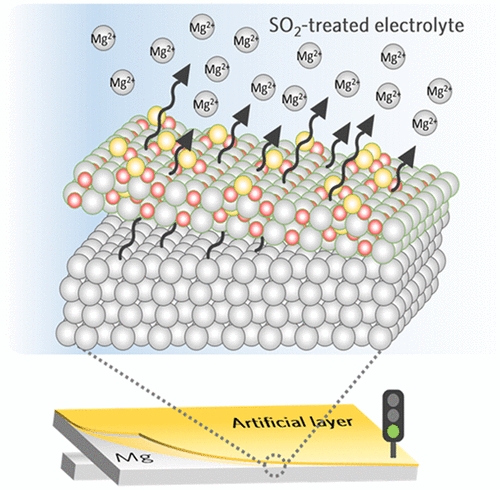
Magnesium (Mg) rechargeable batteries are one of the promising high-energy post-lithium battery chemistries exploiting the multivalent charge carrier. However, the use of magnesium metal has been challenging due to the formation of the ion-blocking passivation layer on magnesium metal in most organic electrolytes. Herein, we propose a new strategy to transform the passivating film into a Mg2+-conductive interphase via simple chemisorption of sulfur dioxide molecules on magnesium metal. The facile chemical tuning converts the magnesium oxide-based passivation layer into a magnesium sulfate-like phase, which greatly enhances the charge-transfer capability of multivalent Mg2+ ions. The reduced surface resistance of the magnesium metal results in efficient magnesium stripping/deposition reactions even under conventional ether-based electrolytes. Theoretical calculations support that the facile ionic conduction is attributed to the relatively low Mg2+ dissociation and migration energies in the tailored interphases. Furthermore, we elucidate the degradation mechanism of magnesium electrodes by combining various experimental analyses with computational calculations.
