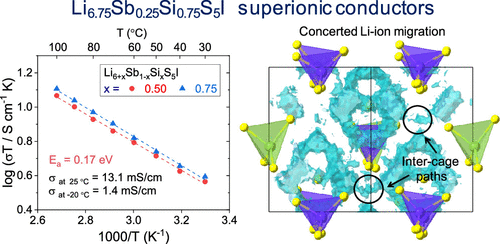
Lithium-based solid electrolytes have been investigated in many studies for improving the energy density and safety of conventional Li-ion batteries. Recently, Li argyrodites (Li6+xSb1–xSixS5I) have been reported as promising superionic conductors, exhibiting an ionic conductivity above 10 mS cm–1. This study examined the high ionic conductivities of Li6+xSb1–xSixS5I using first-principles calculations and subsequent experiments. The calculation results demonstrate that the Li ionic conductivities increase with the Si content in Li6+xSb1–xSixS5I due to the concerted Li-ion migration. Li6+xSb1–xSixS5I compounds synthesized using high-energy ball milling exhibit a high-symmetry argyrodite structure. The Li6.75Sb0.25Si0.75S5I phase demonstrates a favorable combination of a high ionic conductivity of 13.1 mS cm–1 and a low activation energy of 0.17 eV, which was achieved for the first time for cold-pressed pellets, leading to a high ionic conductivity at low temperatures (1.4 mS cm–1 at −20 °C). In addition, Li6.75Sb0.25Si0.75S5I exhibits good electrochemical stability, compatibility with Li metal anodes, high critical current density (1.5 mA cm–2), and hydrolysis stability. Based on the lightweight, low-cost, and non-toxic features of Si, the high Si content in superionic conductor Li6.75Sb0.25Si0.75S5I shows substantial promise for practical use in all-solid-state Li batteries.
