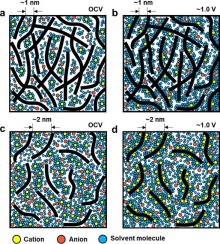
The pseudocapacitive charge storage behavior of redox-active charge carriers has been veiled until now, particularly on nanostructured active carbon materials because of their electrochemical profiles that are similar to typical capacitive behavior and complex carbon structures. In this study, we investigated the origin of the pseudocapacitive reaction using Li-ion charge carriers on nanopore-engineered active carbon materials through both experimental and theoretical analyses. In contrast to the conventional belief that oxygen functional groups are key for pseudocapacitive Li-ion storage behavior, the significant effects of a few nanometer-scale pores are revealed for the first time. The electrochemical profiles of nanoporous carbon, referred to as “ultramesopores,” with a poor oxygen-to-carbon (O/C) ratio of 0.06 exhibited exceptionally high reversible capacities of 840 mA h gelectrode−1 at a cathodic voltage range (1.0–4.8 V vs. Li+/Li). The first-principles calculation results showed that defective carbon structures without any functional groups could be redox hosts for Li-ion storage at a voltage range of 1.5–2.5 V and that the redox voltage was dependent on the domain size of the graphene nanoplatelets. These results show that the pseudocapacitance on nanoporous active carbon materials is affected more by ultramesopores than by oxygen heteroatoms.
