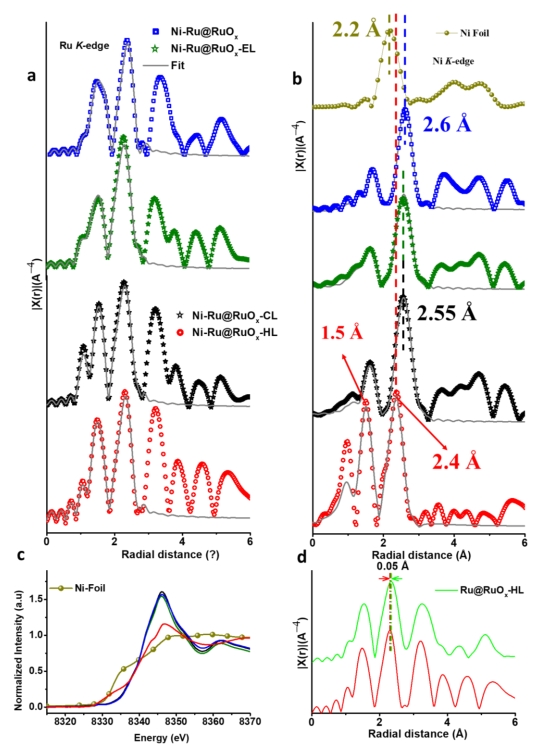
To develop effective electrocatalytic splitting of acidic water, which is a key reaction for renewable energy conversion, the fundamental understanding of sluggish/destructive mechanism of the oxygen evolution reaction (OER) is essential. Through investigating atom/proton/electron transfers in the OER, the distinctive acid–base (AB) and direct-coupling (DC) lattice oxygen mechanisms (LOMs) and adsorbates evolution mechanism (AEM) are elucidated, depending on the surface-defect engineering condition. The designed catalysts are composed of a compressed metallic Ru-core and oxidized Ru-shell with Ni single atoms (SAs). The catalyst synthesized with hot acid treatment selectively follows AB-LOM, exhibiting simultaneously enhanced activity and stability. It produces a current density of 10/100 mA cm−2 at a low overpotential of 184/229 mV and sustains water oxidation at a high current density of up to 20 mA cm−2 over ≈200 h in strongly acidic media.