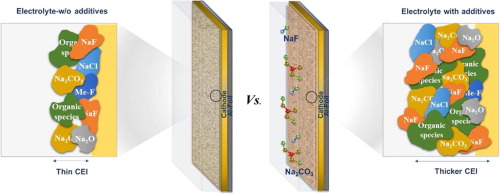
Although Na0.67Fe0.5Mn0.5O2 has attracted tremendous attentions as a cathode material for sodium-ion batteries (NIBs), undesirable side reactions at the interphase between the electrode and electrolyte have limited its wide utilization. An effective way to prevent the side reaction is to artificially induce a mechanically robust and chemically stable cathode electrolyte interphase (CEI). In this paper, functional additives of NaF and Na2CO3 were used to artificially form a thick and stable CEI layer, and their effects were deeply investigated. It was demonstrated that the functional additives in electrolytes could be partially decomposed during a charge process, then it could play a key role in forming a thick and stable interphase directly on the cathode surface. The newly formed CEI layer could sup- press the dissolution of transition metals into the electrolyte and prevent the deterioration of the solid interphase layer. As a result, the additives successfully prevent the capacity fading problem of Na0.67Fe0.5Mn0.5O2 during electrochemical cycling, which results in the improved cyclability compared to the bare Na0.67Fe0.5Mn0.5O2. We believe that the addition of functional additive is a simple and cost-effective way to artificially form a stable CEI layer on a cathode, therefore, this approach is expected to be widely applicable to other electrode materials that suffer from the unstable interfaces.
