Stabilization of Oxygen-Dependent Fe3+/4+ Redox in Li-Excess DRX Cathode Exhibiting Anionic Redox via Transition Metal Combination
- Year
- 2024
- Author
- Hayeon Lee, Minji Kim, Hyunyoung Park, Yiseul Yoo, Sangmun Na, Hee-Dae Lim, Jongsoon Kim, Won-Sub Yoon*
- Journal
- Advanced Functional Materials
- Vol
- 34
- Page
- 2312401
- Link
- https://doi.org/10.1002/adfm.202312401 584회 연결
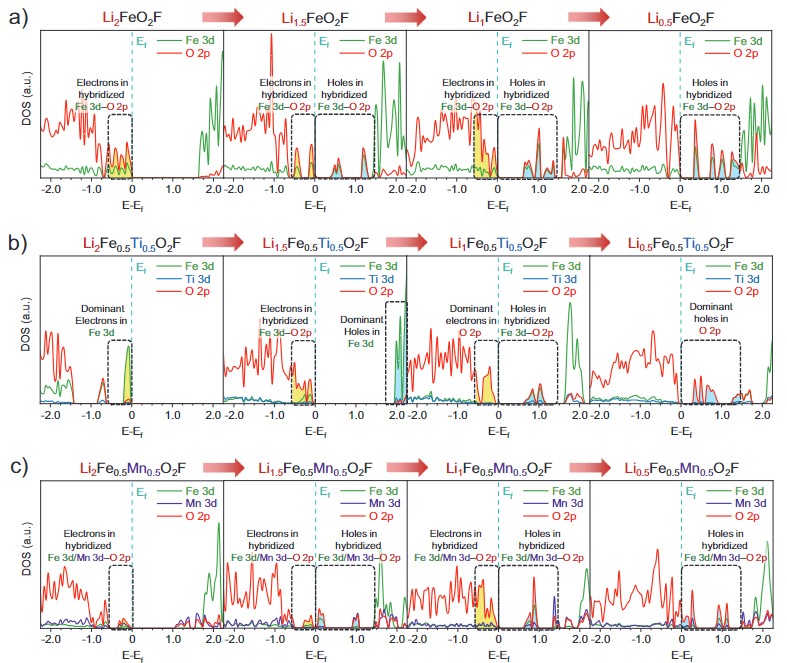
Developing sustainable Li-ion batteries requires high-energy cathodes based on low-cost, earth-abundant elements, moving away from low-reserve nickel and cobalt. Fe-based oxide cathodes with Fe3+/4+ and O2−/n− redox couples offer potential but face low initial Coulombic efficiency and significant voltage hysteresis. This study investigates Li-excess Fe-based disordered rock-salt (DRX) oxyfluorides (Li2Fe0.5M0.5O2F; M = Fe, Ti, Mn) using combined electrochemical/spectroscopic characterization and first-principles calculation. Oxygen-dependent Fe3+/4+ redox, related to Fe 3d–O 2p hybrid state, can be stabilized when combined with Mn3+/4+ redox in DRX structure owing to the unusual decrease in its redox potential. The moderately high charge transfer gap stabilizes Fe4+ against ligand-to-metal charge transfer (LMCT) on charge, reduces the amount of oxygen oxidation, thereby increasing Coulombic efficiency. On discharge, it allows metal-to-ligand charge transfer (MLCT) without substantial overpotential, reducing hysteresis in oxygen redox. The resulting composition exhibits high capacity (309 mAh g−1) and energy density (998 Wh kg−1), providing insights for next-generation Ni- and Co-free cathode materials.